ccl4 polar or nonpolar
Hence chlorine is a non-polar molecule. Does CCl4 contain polar bonds.
 |
| Makethebrainhappy Is Ccl4 Polar Or Nonpolar |
Due to an electronegativity difference of 061 units between bonded C and Cl atoms each C-Cl bond in the CCl 4 molecule.
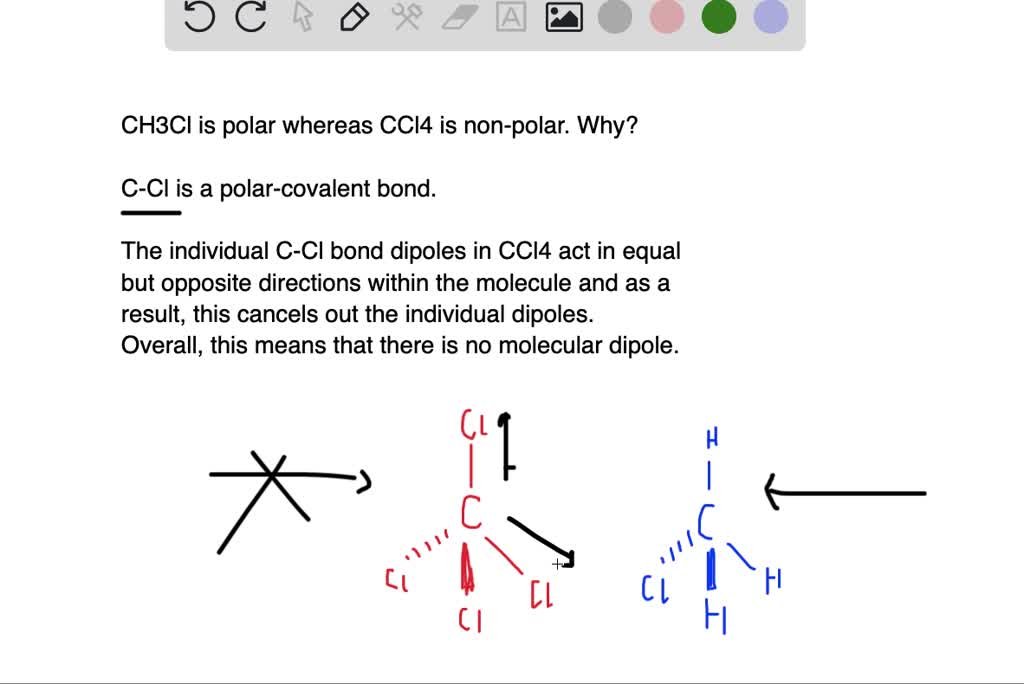
. Hey Guys In this video we are going to determine the polarity of Carbon Tetrachloride having a chemical formula of CCl4. CCl4 and BF3 are nonpolar molecules. From this information alone it can be deduced that. In ccl4 the central atom is carbon bearing atomic no.
But the CCl 4 molecule is a. CCl4 or Carbon tetrachloride is a NONPOLAR molecule because all the four bonds C-Cl bonds are identical and CCl4 has symmetrical geometry which cancels out the bond. Carbon tetrachloride is a polar molecule because it has a permanent dipole. There is no net dipole and the CCl4 is non-polar.
So compounds have a net-zero dipole. Screen capture done with Camtasia. Lets dive into it. So now we have to study the formation and how the molecule is polar and nonpolar in chemistry.
6and in ccl4 it is sp3 hybridisationso ccl4 has a tetrahedral structure. Since the electronegativity difference is 04 which lies between 04 and 17. However the C-Cl bond is a polar covalent bond but the four bonds cancel the polarity of each. CH4 or Methane is a NONPOLAR molecule because all the four bonds C-H bonds are identical and CH4 has symmetrical geometry.
The SiCl4 molecule is nonpolar and chlorine is more electronegative than silicon. The molecule of CCl4 is nonpolar in nature because of its symmetrical tetrahedral structure. However the C-Cl bond is a polar covalent bond but the four bonds cancel the polarity of each. The four bonds of carbon tetrachloride CCl4 are polar but the molecule is nonpolar because the.
1 SiCl bond is nonpolar. This means that one side of the molecule is positively charged and the other side is negatively charged. It is made of one Carbon atom and. The molecule of CCl4 is nonpolar in nature because of its symmetrical tetrahedral structure.
The vector is equal in magnitude and opposite in direction Therefore the dipole moment of one bond cancels the other bond placed opposite to it. The C Cl bond will have a polar covalent character. However the C-Cl bond is a polar covalent bond but the four. HCl andH20 is polar molecules.
The molecule of CCl4 is nonpolar in nature because of its symmetrical tetrahedral structure. Why is CCl4 not a polar molecule remember to think about the bonds inside the molecule and about symmetry. 4 230 reviews Highest rating. DO NOT FORGET TO SUBSCRIBELinkedIn.
Only the C Cl bond is polar. Carbon tetrachloride CCl 4 is a non-polar molecule with µ0. The four bonds of carbon tetrachloride CCl4 are polar but themolecule is nonpolar because the bond polarity is canceled by thesymmetric tetrahedral shape.
 |
| Ccl4 Lewis Structure How To Draw The Dot Structure For Ccl4 Carbon Tetachloride Youtube |
 |
| Assertion C Cl 4 Is A Non Polar Molecule Reason C Cl 4 Has Polar Bonds Youtube |
 |
| Kelompokkan Senyawa Berikut Manakah Yang Termasuk Senyaw |
 |
| Ch4 Ccl4 C2h2 Co2 Kenapa Disebut Senyawa Non Polar Padahal Ada Unsur Yang Memiliki Jelaskan Brainly Co Id |
 |
| The Carbon Tetrachloride Molecule Ccl4 Is A A Nonpolar Molecule With Polar Bonds B A Nonpolar Molecule With Nonpolar Bonds C A Polar Molecule With Polar Bonds D A Polar Molecule With |
Posting Komentar untuk "ccl4 polar or nonpolar"